Neurotransmitter transport into synaptic vesicles
The regulated exocytosis of specialized secretory vesicles confers the quantal nature of neurotransmission. This mode of signaling depends on the transport of transmitter from the cytoplasm into secretory vesicles. In contrast to plasma membrane transporters that terminate signaling and recycle transmitter, vesicle transporters are required for quantal release. The lumenal concentration of transmitter also determines the extent of receptor activation, which receptors are activated (low as well as high affinity) and their distance from the site of release. Despite their importance, the intracellular location of vesicular neurotransmitter transporters has made it difficult to understand the mechanisms involved and their potential for regulation. Radiotracer flux assays have shown that synaptic vesicles accumulate all of the classical transmitters. All of these transport activities depend on a H+ electrochemical gradient, but the vesicular monoamine and acetylcholine transporters depend primarily on the chemical component of this gradient (the pH gradient) whereas the vesicular glutamate transporters depend predominantly on membrane potential and the vesicular GABA transporter more equally on both. This raises the question whether expression of the H+ electrochemical gradient as chemical or electrical gradients can be regulated and if so, how? And how do these properties adapted to the release of different transmitters?
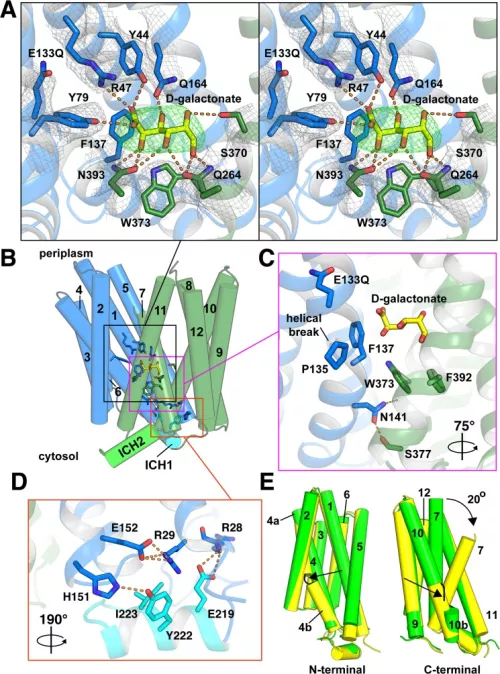
In recent years, we have focused on the mechanism of vesicular glutamate transport due to its essential role in excitatory neurotransmission. To understand the function of the vesicular glutamate transporters (VGLUTs), we use a variety of assays (including some that we have developed), from uptake by synaptic vesicles to recording the associated currents in heterologous expression systems (Xenopus oocytes and HEK cells) and transport by purified VGLUT reconstituted into proteoliposomes. Through this work, we have found that the VGLUTs undergo allosteric regulation by protons and chloride from the lumen of the synaptic vesicle, and exhibit an associated chloride conductance gated by the same mechanisms (Fig. on left side). Although the physiological significance of this regulation has not been established, it seems likely to coordinate vesicle filling with the massive ionic shifts that accompany the exocytosis and rapid recycling of synaptic vesicles. We have also pursued the structural analysis of this transporter family, recently reporting two structures of a related family member from bacteria (Fig. on the right side). Although very closely related in sequence to the VGLUTs, the bacterial protein and other mammalian proteins in the same family exhibit major differences in function that illuminate mechanisms regulating the VGLUTs.
Eriksen J, Chang R, McGregor M, Silm K, Suzuki T, Edwards RH. Protons Regulate Vesicular Glutamate Transporters through an Allosteric Mechanism. Neuron. 2016 05 18; 90(4):768-80. PMID: 27133463.
Leano JB, Batarni S, Eriksen J, Juge N, Pak JE, Kimura-Someya T, Robles-Colmenares Y, Moriyama Y, Stroud RM, Edwards RH. Structures suggest a mechanism for energy coupling by a family of organic anion transporters. PLoS Biol. 2019 05; 17(5):e3000260. PMID: 31083648
Neurotransmitter corelease
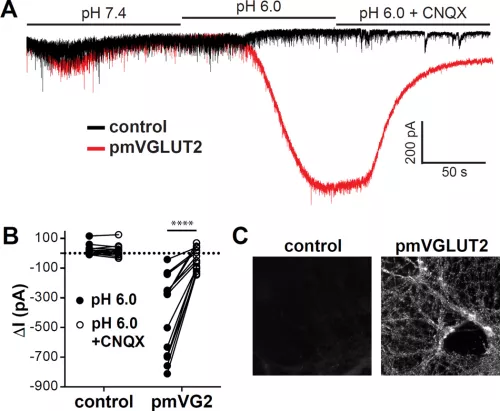
The localization of vesicular neurotransmitter transporters defines the cells and indeed the intracellular membranes that are capable of regulated release by exocytosis. We have thus used these transporters to study the phenomenon of neurotransmitter corelease. Focusing on glutamate corelease by dopamine neurons, we have found evidence for both colocalization and segregation of VGLUT2 and the vesicular monoamine transporter VMAT2. In vesicles with both transporters, the two transmitters appear to promote filling by the other, apparently through synergistic effects on the H+-ATPase that provides the H+ electrochemical driving force. However, we have also found that the two transmitters are released with different properties, indicating molecular differences among synaptic vesicles from the same cell. In contrast to the high probability of glutamate release that depresses with repeated stimulation, dopamine release has a lower probability and facilitates. The two transmitters thus extract different information from the neural firing rate, predicting distinct roles for the two transmitters in the tonic and burst firing of dopamine neurons. The mechanism reflects at least in part coupling to different presynaptic calcium channels. We have also found that different endocytic mechanisms are responsible for generating these two synaptic vesicle populations. We are now characterizing the molecular basis for these differences in release and the mechanisms that regulate them.
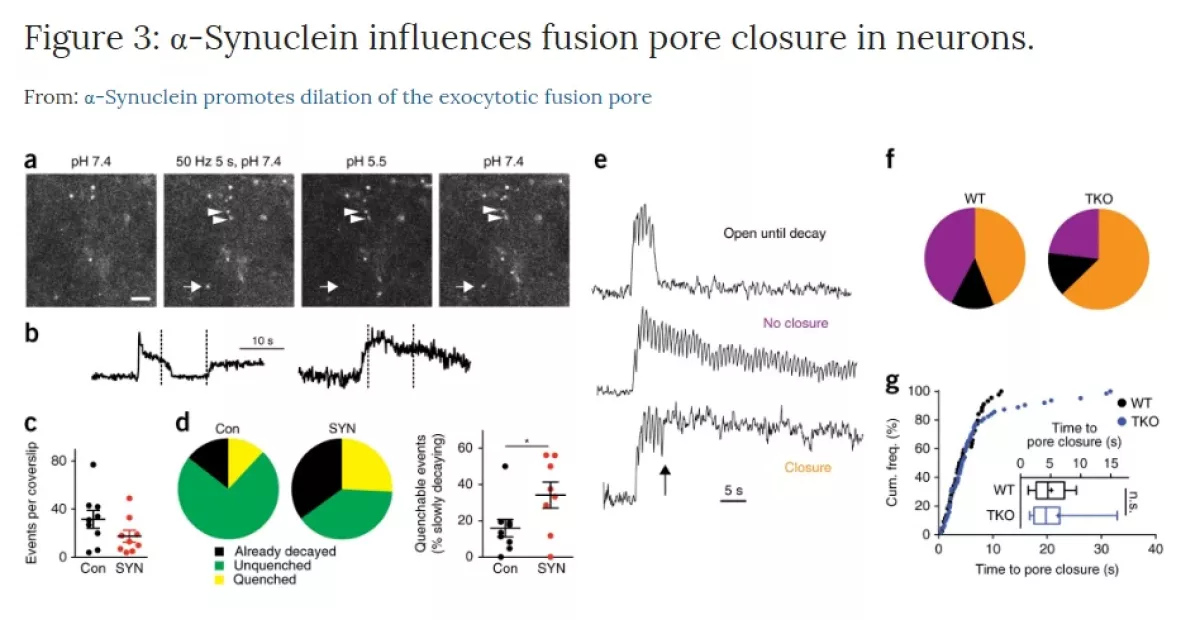
α-Synuclein and the fusion pore
We originally identified the vesicular monoamine transporter by virtue of its ability to protect cells against the parkinsonian neurotoxin MPP+. This predicted a role for regulated exocytosis in the pathogenesis of Parkinson’s disease (PD) that we continue to explore. We now study the presynaptic role of alpha-synuclein, a protein with a central, causative role in PD. We have found that over-expression of synuclein inhibits synaptic vesicle exocytosis, but the analysis of knockout mice shows that endogenous synuclein serves to promote dilation of the pore that forms during membrane fusion. Using this function as an assay, we are now exploring the structural basis for this activity and testing the effect of both disease mutations and other factors implicated in PD. The goal is to understand the normal biology of synuclein so that we can understand the first steps that lead to degeneration.
Biogenesis of large dense core vesicles
In contrast to small synaptic vesicles that release classical neurotransmitters, large dense core vesicles release peptide hormones (such as insulin), neural peptides and some growth factors. However, the mechanism by which they acquire the capacity for regulated exocytosis has remained a major question in cell biology. We have recently identified some of the first components of the cytosolic machinery that produce dense core vesicles, and are now using them to explore this process central to organismal physiology.